The equipment planned to help bring samples back from Mars.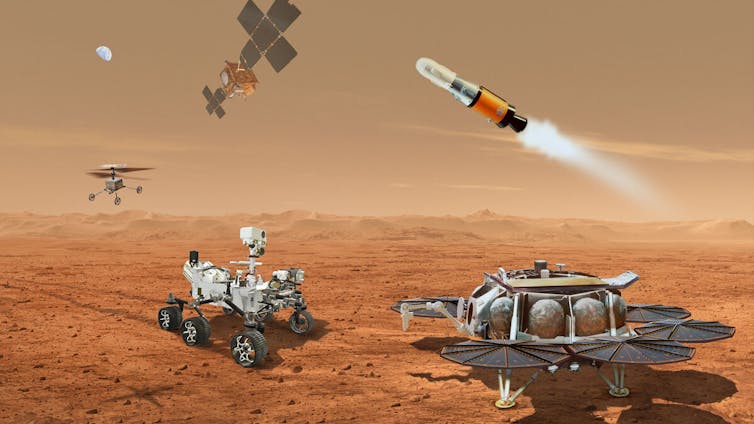
Chris Impey, University of Arizona
A critical NASA mission in the search for life beyond Earth, Mars Sample Return, is in trouble. Its budget has ballooned from US$5 billion to over $11 billion, and the sample return date may slip from the end of this decade to 2040.
The mission would be the first to try to return rock samples from Mars to Earth so scientists can analyze them for signs of past life.
NASA Administrator Bill Nelson said during a press conference on April 15, 2024, that the mission as currently conceived is too expensive and too slow. NASA gave private companies a month to submit proposals for bringing the samples back in a quicker and more affordable way.
As an astronomer who studies cosmology and has written a book about early missions to Mars, I’ve been watching the sample return saga play out. Mars is the nearest and best place to search for life beyond Earth, and if this ambitious NASA mission unraveled, scientists would lose their chance to learn much more about the red planet.
The habitability of Mars
The first NASA missions to reach the surface of Mars in 1976 revealed the planet as a frigid desert, uninhabitable without a thick atmosphere to shield life from the Sun’s ultraviolet radiation. But studies conducted over the past decade suggest that the planet may have been much warmer and wetter several billion years ago.
The Curiosity and Perseverance rovers have each shown that the planet’s early environment was suitable for microbial life.
They found the chemical building blocks of life and signs of surface water in the distant past. Curiosity, which landed on Mars in 2012, is still active; its twin, Perseverance, which landed on Mars in 2021, will play a crucial role in the sample return mission.
The Mars Jezero Crater, which scientists are searching for signs of ancient bacteria.
Why astronomers want Mars samples
The first time NASA looked for life in a Mars rock was in 1996. Scientists claimed they had discovered microscopic fossils of bacteria in the Martian meteorite ALH84001. This meteorite is a piece of Mars that landed in Antarctica 13,000 years ago and was recovered in 1984. Scientists disagreed over whether the meteorite really had ever harbored biology, and today most scientists agree that there’s not enough evidence to say that the rock contains fossils.
Several hundred Martian meteorites have been found on Earth in the past 40 years. They’re free samples that fell to Earth, so while it might seem intuitive to study them, scientists can’t tell where on Mars these meteorites originated. Also, they were blasted off the planet’s surface by impacts, and those violent events could have easily destroyed or altered subtle evidence of life in the rock.
There’s no substitute for bringing back samples from a region known to have been hospitable to life in the past. As a result, the agency is facing a price tag of $700 million per ounce, making these samples the most expensive material ever gathered.
A compelling and complex mission
Bringing Mars rocks back to Earth is the most challenging mission NASA has ever attempted, and the first stage has already started.
Perseverance has collected over two dozen rock and soil samples, depositing them on the floor of the Jezero Crater, a region that was probably once flooded with water and could have harbored life. The rover inserts the samples in containers the size of test tubes. Once the rover fills all the sample tubes, it will gather them and bring them to the spot where NASA’s Sample Retrieval Lander will land. The Sample Retrieval Lander includes a rocket to get the samples into orbit around Mars.

The European Space Agency has designed an Earth Return Orbiter, which will rendezvous with the rocket in orbit and capture the basketball-sized sample container. The samples will then be automatically sealed into a biocontainment system and transferred to an Earth entry capsule, which is part of the Earth Return Orbiter. After the long trip home, the entry capsule will parachute to the Earth’s surface.
The complex choreography of this mission, which involves a rover, a lander, a rocket, an orbiter and the coordination of two space agencies, is unprecedented. It’s the culprit behind the ballooning budget and the lengthy timeline.
Sample return breaks the bank
Mars Sample Return has blown a hole in NASA’s budget, which threatens other missions that need funding.
The NASA center behind the mission, the Jet Propulsion Laboratory, just laid off over 500 employees. It’s likely that Mars Sample Return’s budget partly caused the layoffs, but they also came down to the Jet Propulsion Laboratory having an overfull plate of planetary missions and suffering budget cuts.
Within the past year, an independent review board report and a report from the NASA Office of Inspector General raised deep concerns about the viability of the sample return mission. These reports described the mission’s design as overly complex and noted issues such as inflation, supply chain problems and unrealistic costs and schedule estimates.
NASA is also feeling the heat from Congress. For fiscal year 2024, the Senate Appropriations Committee cut NASA’s planetary science budget by over half a billion dollars. If NASA can’t keep a lid on the costs, the mission might even get canceled.
Thinking out of the box
Faced with these challenges, NASA has put out a call for innovative designs from private industry, with a goal of shrinking the mission’s cost and complexity. Proposals are due by May 17, which is an extremely tight timeline for such a challenging design effort. And it’ll be hard for private companies to improve on the plan that experts at the Jet Propulsion Laboratory had over a decade to put together.
An important potential player in this situation is the commercial space company SpaceX. NASA is already partnering with SpaceX on America’s return to the Moon. For the Artemis III mission, SpaceX will attempt to land humans on the Moon for the first time in more than 50 years.
However, the massive Starship rocket that SpaceX will use for Artemis has had only three test flights and needs a lot more development before NASA will trust it with a human cargo.
SpaceX’s Starship rocket, the most powerful commercial rocket.
In principle, a Starship rocket could bring back a large payload of Mars rocks in a single two-year mission and at far lower cost. But Starship comes with great risks and uncertainties. It’s not clear whether that rocket could return the samples that Perseverance has already gathered.
Starship uses a launchpad, and it would need to be refueled for a return journey. But there’s no launchpad or fueling station at the Jezero Crater. Starship is designed to carry people, but if astronauts go to Mars to collect the samples, SpaceX will need a Starship rocket that’s even bigger than the one it has tested so far.
Sending astronauts also carries extra risk and cost, and a strategy of using people might end up more complicated than NASA’s current plan.
With all these pressures and constraints, NASA has chosen to see whether the private sector can come up with a winning solution. We’ll know the answer next month.![]()
Chris Impey, University Distinguished Professor of Astronomy, University of Arizona
This article is republished from The Conversation under a Creative Commons license. Read the original article.