How did early cells keep themselves distinct while allowing for some amount of exchange? UChicago Pritzker School of Molecular Engineering/Peter Allen, Second Bay Studios, CC BY-ND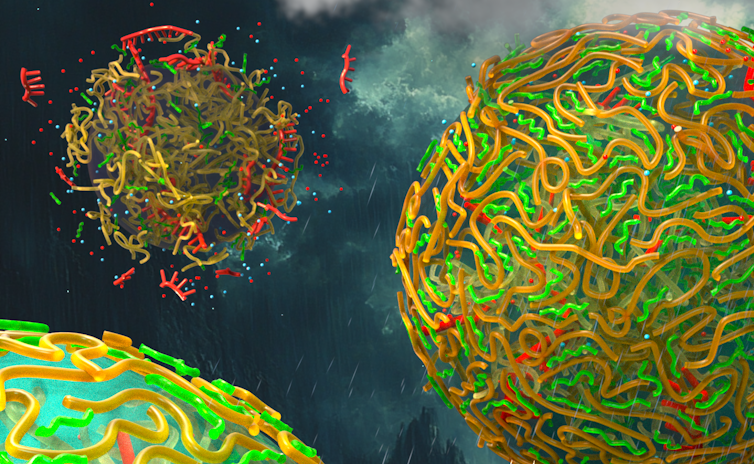
Aman Agrawal, University of Chicago Pritzker School of Molecular Engineering
Billions of years of evolution have made modern cells incredibly complex. Inside cells are small compartments called organelles that perform specific functions essential for the cell’s survival and operation. For instance, the nucleus stores genetic material, and mitochondria produce energy.
Another essential part of a cell is the membrane that encloses it. Proteins embedded on the surface of the membrane control the movement of substances in and out of the cell. This sophisticated membrane structure allowed for the complexity of life as we know it. But how did the earliest, simplest cells hold it all together before elaborate membrane structures evolved?
In our recently published research in the journal Science Advances, my colleagues from the University of Chicago and the University of Houston and I explored a fascinating possibility that rainwater played a crucial role in stabilizing early cells, paving the way for life’s complexity.
The origin of life
One of the most intriguing questions in science is how life began on Earth. Scientists have long wondered how nonliving matter like water, gases and mineral deposits transformed into living cells capable of replication, metabolism and evolution.
Chemists Stanley Miller and Harold Urey at the University of Chicago conducted an experiment in 1953 demonstrating that complex organic compounds – meaning carbon-based molecules – could be synthesized from simpler organic and inorganic ones. Using water, methane, ammonia, hydrogen gases and electric sparks, these chemists formed amino acids.
The Miller-Urey experiment showed that complex organic compounds can be made from simpler organic and inorganic materials.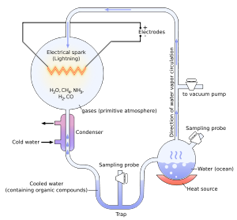
Scientists believe the earliest forms of life, called protocells, spontaneously emerged from organic molecules present on the early Earth. These primitive, cell-like structures were likely made of two fundamental components: a matrix material that provided a structural framework and a genetic material that carried instructions for protocells to function.
Over time, these protocells would have gradually evolved the ability to replicate and execute metabolic processes. Certain conditions are necessary for essential chemical reactions to occur, such as a steady energy source, organic compounds and water. The compartments formed by a matrix and a membrane crucially provide a stable environment that can concentrate reactants and protect them from the external environment, allowing the necessary chemical reactions to take place.
Thus, two crucial questions arise: What materials were the matrix and membrane of protocells made of? And how did they enable early cells to maintain the stability and function they needed to transform into the sophisticated cells that constitute all living organisms today?
Bubbles vs droplets
Scientists propose that two distinct models of protocells – vesicles and coacervates – may have played a pivotal role in the early stages of life.
Miniature compartments, such as lipid bilayers configured into capsules like liposomes and micelles, are important for cellular organization and function.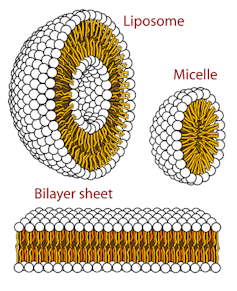
Vesicles are tiny bubbles, like soap in water. They are made of fatty molecules called lipids that naturally form thin sheets. Vesicles form when these sheets curl into a sphere that can encapsulate chemicals and safeguard crucial reactions from harsh surroundings and potential degradation.
Like miniature pockets of life, vesicles resemble the structure and function of modern cells. However, unlike the membranes of modern cells, vesicle protocells would have lacked specialized proteins that selectively allow molecules in and out of a cell and enable communication between cells. Without these proteins, vesicle protocells would have limited ability to interact effectively with their surroundings, constraining their potential for life.
Coacervates, on the other hand, are droplets formed from an accumulation of organic molecules like peptides and nucleic acids. They form when organic molecules stick together due to chemical properties that attract them to each other, such as electrostatic forces between oppositely charged molecules. These are the same forces that cause balloons to stick to hair.
One can picture coacervates as droplets of cooking oil suspended in water. Similar to oil droplets, coacervate protocells lack a membrane. Without a membrane, surrounding water can easily exchange materials with protocells. This structural feature helps coacervates concentrate chemicals and speed up chemical reactions, creating a bustling environment for the building blocks of life.
Thus, the absence of a membrane appears to make coacervates a better protocell candidate than vesicles. However, lacking a membrane also presents a significant drawback: the potential for genetic material to leak out.
Unstable and leaky protocells
A few years after Dutch chemists discovered coacervate droplets in 1929, Russian biochemist Alexander Oparin proposed that coacervates were the earliest model of protocells. He argued that coacervate droplets provided a primitive form of compartmentalization crucial for early metabolic processes and self-replication.
Subsequently, scientists discovered that coacervates can sometimes be composed of oppositely charged polymers: long, chainlike molecules that resemble spaghetti at the molecular scale, carrying opposite electrical charges. When polymers of opposite electrical charges are mixed, they tend to attract each other and stick together to form droplets without a membrane.
Coacervate droplets resemble oil suspended in water. Aman Agrawal, CC BY-SA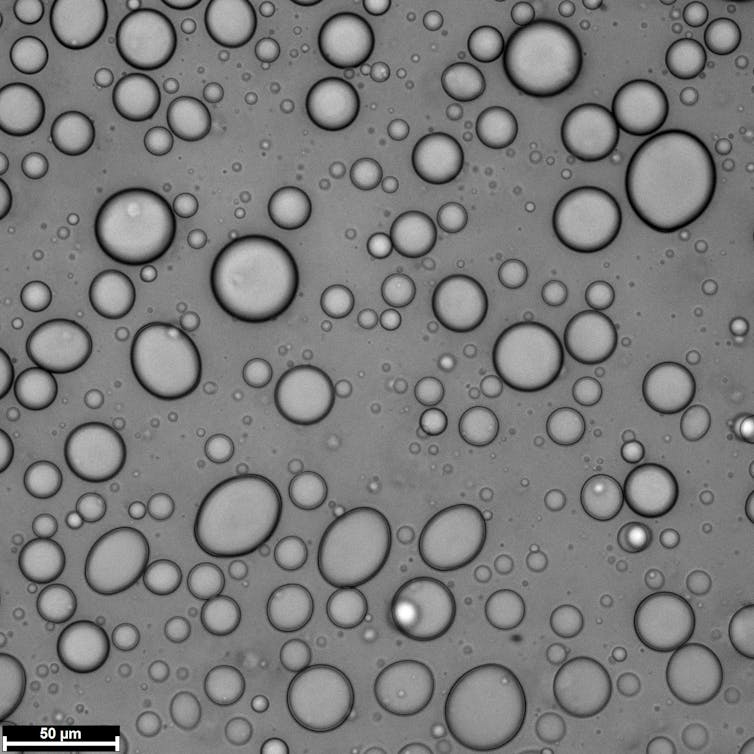
The absence of a membrane presented a challenge: The droplets rapidly fuse with each other, akin to individual oil droplets in water joining into a large blob. Furthermore, the lack of a membrane allowed RNA – a type of genetic material thought to be the earliest form of self-replicating molecule, crucial for the early stages of life – to rapidly exchange between protocells.
My colleague Jack Szostak showed in 2017 that rapid fusion and exchange of materials can lead to uncontrolled mixing of RNA, making it difficult for stable and distinct genetic sequences to evolve. This limitation suggested that coacervates might not be able to maintain the compartmentalization necessary for early life.
Compartmentalization is a strict requirement for natural selection and evolution. If coacervate protocells fused incessantly, and their genes continuously mixed and exchanged with each other, all of them would resemble each other without any genetic variation. Without genetic variation, no single protocell would have a higher probability of survival, reproduction and passing on its genes to future generations.
But life today thrives with a variety of genetic material, suggesting that nature somehow solved this problem. Thus, a solution to this problem had to exist, possibly hiding in plain sight.
Rainwater and RNA
A study I conducted in 2022 demonstrated that coacervate droplets can be stabilized and avoid fusion if immersed in deionized water – water that is free of dissolved ions and minerals. The droplets eject small ions into the water, likely allowing oppositely charged polymers on the periphery to come closer to each other and form a meshy skin layer. This meshy “wall” effectively hinders the fusion of droplets.
Next, with my colleagues and collaborators, including Matthew Tirrell and Jack Szostak, I studied the exchange of genetic material between protocells. We placed two separate protocell populations, treated with deionized water, in test tubes. One of these populations contained RNA. When the two populations were mixed, RNA remained confined in their respective protocells for days. The meshy “walls” of the protocells impeded RNA from leaking.
In contrast, when we mixed protocells that weren’t treated with deionized water, RNA diffused from one protocell to the other within seconds.
Inspired by these results, my colleague Alamgir Karim wondered if rainwater, which is a natural source of ion-free water, could have done the same thing in the prebiotic world. With another colleague, Anusha Vonteddu, I found that rainwater indeed stabilizes protocells against fusion.
Rain, we believe, may have paved the way for the first cells.
Droplets with meshy walls resist fusion and prevent leakage of their RNA. In this image, each color represents a different type of RNA. Aman Agrawal, CC BY-SA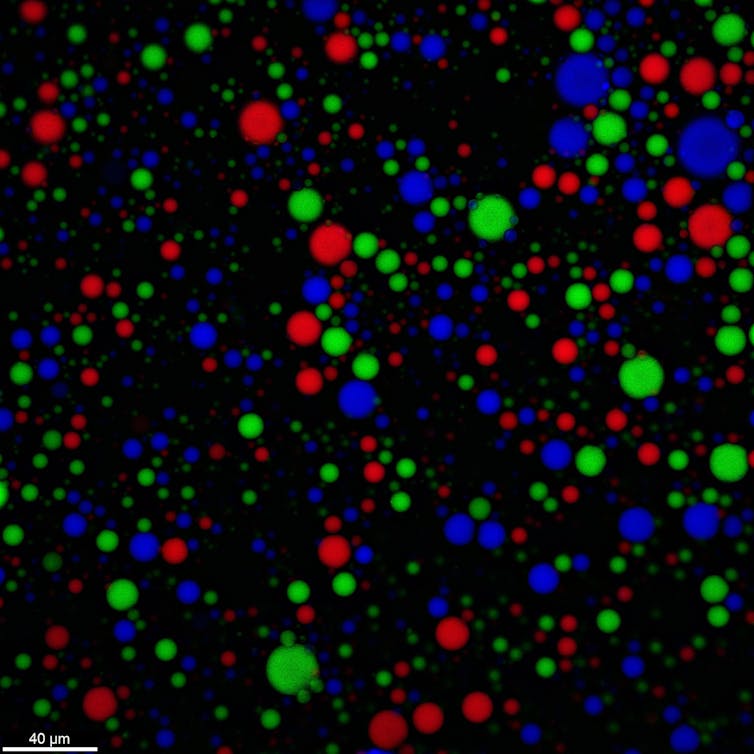
Working across disciplines
Studying the origins of life addresses both scientific curiosity about the mechanisms that led to life on Earth and philosophical questions about our place in the universe and the nature of existence.
Currently, my research delves into the very beginning of gene replication in protocells. In the absence of the modern proteins that make copies of genes inside cells, the prebiotic world would have relied on simple chemical reactions between nucleotides – the building blocks of genetic material – to make copies of RNA. Understanding how nucleotides came together to form a long chain of RNA is a crucial step in deciphering prebiotic evolution.
To address the profound question of life’s origin, it is crucial to understand the geological, chemical and environmental conditions on early Earth approximately 3.8 billion years ago. Thus, uncovering the beginnings of life isn’t limited to biologists. Chemical engineers like me, and researchers from various scientific fields, are exploring this captivating existential question.![]()
Aman Agrawal, Postdoctoral Scholar in Chemical Engineering, University of Chicago Pritzker School of Molecular Engineering
This article is republished from The Conversation under a Creative Commons license. Read the original article.