Kaiser Health News
Trump Official Who OK’d Drugs From Canada Chairs Company Behind Florida’s Import Plan
Phil Galewitz, KFF Health News
Fri, 12 Jan 2024 19:20:00 +0000
The Food and Drug Administration’s unprecedented approval of Florida’s plan to import drugs from Canada was made possible only after Alex Azar, as the Trump administration’s Health and Human Services secretary, certified that bringing medicines over the border could be done safely.
Azar made the historic declaration in September 2020, just two months before his boss, former President Donald Trump, lost reelection.
Now, Azar’s involved in the business of making importation happen. He is chairman of the board of LifeScience Logistics, a Dallas-based company that Florida is paying as much as $39 million to help manage its Canadian drug importation program, not including the cost of drugs.
LifeScience officials confirmed Azar’s position but didn’t respond to questions about how much he is paid or whether he’s involved in the Florida work. Azar didn’t return messages left with his employers or sent to a personal email address.
The revolving door between government and private sector jobs is well documented. It’s common for top U.S. officials in both parties to leave government service for what are often far better-paid jobs or board seats at companies in the industries they formerly regulated.
About 57% of presidential Cabinet-level officials later served on corporate boards of directors, according to a 2019 study by researchers at Boston and Harvard universities in The Journal of Politics, which examined 84 Cabinet members who served from 1992 to 2014.
“In general, we favor Cabinet secretaries not going into industries which they once regulated, because the possibility of conflicts of interest are unavoidable,” said Robert Weissman, president of Public Citizen, a government watchdog group.
He called Azar’s case atypical because his approval of drug importation was opposed by the pharmaceutical industry, in which Azar was formerly employed. Drugmakers argue the policy puts patients at risk of consuming counterfeit medicines. Azar joined the LifeScience board in January 2022, one year after the end of Trump’s term and about a year after Florida contracted with LifeScience in late 2020.
Katie Hernandez, a spokesperson for LifeScience Logistics, said in a statement that the company, which manages nearly 6 million square feet of warehouse storage across 11 states, signed its deal with Florida before Azar joined the board.
Ivana Katic, assistant professor of organizational behavior at the Yale School of Management, said that Azar’s position at LifeScience “can appear as a conflict of interest” because his policy decision as HHS secretary later benefited him professionally.
Azar was a deputy secretary at HHS during the George W. Bush administration before joining pharmaceutical giant Eli Lilly and Co. as a top executive in 2007, remaining there until months before joining the Trump administration.
Weissman, who supports drug importation, said he doubts Azar had any personal benefit in mind before his decision. Florida Gov. Ron DeSantis had pushed Trump to authorize importation from Canada, and the former president had said he supported importation before Azar certified it was safe.
Canadian drug importation has been the subject of decades of debate. While the U.S. does not regulate most drug costs, Canada does, generally resulting in lower prices than across the border.
In 2018, Azar called importation a “gimmick” because Canada’s pharmaceutical market isn’t large enough to meet U.S. demand. Indeed, the Canadian government has repeatedly warned the U.S. against importation, promising to block any plan that poses a risk of causing shortages in Canada.
The country has implemented regulations “to prohibit certain drugs intended for the Canadian market from being sold for consumption outside of Canada if that sale could cause, or worsen, a drug shortage in Canada,” Health Canada, which regulates drug safety, said in a Jan. 8 statement after the FDA’s approval of Florida’s plan. “This includes all drugs that are eligible for bulk importation to the U.S., including those identified in Florida’s bulk importation plan, or any other US state’s future importation programs.”
Under its contract with Florida, LifeScience Logistics must buy drugs from Canadian suppliers, contract with a lab to verify their authenticity, store the medicines, and ship them to state agencies for distribution. LifeScience built a 100,000-square-foot facility in Lakeland, Florida, to warehouse drugs imported from Canada.
President Joe Biden supported drug importation during his 2020 campaign, but after the election his administration moved slowly to advance the process. Colorado has an importation application pending with the FDA, while several other states have passed laws allowing for importation. DeSantis has accused the Biden administration of slow-walking a decision, and his administration filed a lawsuit over the FDA’s delay.
Florida’s importation plan will save the state up to $180 million in the first year of the program, the state said. The importation program wouldn’t aid consumers directly. It’s instead aimed at helping state agencies, including its prisons, health department, and Medicaid program, obtain lower-cost drugs for HIV and AIDS, diabetes, and other conditions.
Florida’s plan still faces many hurdles. On top of Canada’s reluctance to participate in U.S. importation programs, some drug manufacturers have deals with Canadian wholesalers preventing them from exporting medicines, and the FDA decision is likely to face a legal challenge by drugmakers.
The drug industry’s major lobbying group, the Pharmaceutical Research and Manufacturers of America, or PhRMA, previously sued to stop Azar’s importation decision. It’s expected to file suit to block Florida’s program as well.
A PhRMA spokesperson declined to comment on Azar’s role.
——————————
By: Phil Galewitz, KFF Health News
Title: Trump Official Who OK’d Drugs From Canada Chairs Company Behind Florida’s Import Plan
Sourced From: kffhealthnews.org/news/article/alex-azar-hhs-secretary-canadian-drug-importation-company-board/
Published Date: Fri, 12 Jan 2024 19:20:00 +0000
Kaiser Health News
Readers Embrace ‘Going It Alone’ Series on Aging and Chastise Makers of Pulse Oximeters
SUMMARY: Letters to the Editor discuss various healthcare concerns. Gail Daniels shares her struggles caring for a mother with dementia, while Shava Nerad reflects on the challenges faced by those without family support. Gloria Rankin suggests using pen pals to combat social isolation. Zoe Joyner Danielson recalls racial bias in pulse oximeter development, while Suzann Lebda questions fluoride’s impact on dental health. Readers also address issues like Medicare Advantage, high drug costs for seniors, and the financial burden of prepaying for baby deliveries. Liviu Steier advocates for fluorescence in dental care, emphasizing its diagnostic benefits.
The post Readers Embrace ‘Going It Alone’ Series on Aging and Chastise Makers of Pulse Oximeters appeared first on kffhealthnews.org
Kaiser Health News
Georgians With Disabilities Are Still Being Institutionalized, Despite Federal Oversight
SUMMARY: Lloyd Mills, a 32-year-old with autism, cerebral palsy, and kidney disease, has faced prolonged hospitalization due to inadequate community support in Georgia. After being admitted to Grady Memorial Hospital for mental health issues, Mills waited over eight months for appropriate housing, highlighting the systemic failures of a state still grappling with the consequences of a 2010 Department of Justice lawsuit regarding care for people with developmental disabilities. Despite significant investments and improvements in services, challenges like workforce shortages and inadequate funding persist, often leaving individuals like Mills in hospitals, impacting their mental and physical well-being.
The post Georgians With Disabilities Are Still Being Institutionalized, Despite Federal Oversight appeared first on kffhealthnews.org
Kaiser Health News
TV’s Dr. Oz Invested in Businesses Regulated by Agency Trump Wants Him To Lead
SUMMARY: President-elect Donald Trump nominated celebrity doctor Mehmet Oz to head the Centers for Medicare & Medicaid Services (CMS). Oz, known for his investments in healthcare, tech, and food companies, holds significant stakes in UnitedHealth Group, CVS Health, Amazon, and other companies involved in health insurance and pharmaceuticals, raising potential conflicts of interest. His financial ties include hospital stocks and pharmaceutical investments. Oz has expressed support for Medicare Advantage and criticized the food and healthcare industries. Critics question whether Oz can separate his financial interests from his role, particularly with companies doing business with the federal government.
The post TV’s Dr. Oz Invested in Businesses Regulated by Agency Trump Wants Him To Lead appeared first on kffhealthnews.org
-

 Kaiser Health News5 days ago
Kaiser Health News5 days agoA Closely Watched Trial Over Idaho’s Near-Total Abortion Ban Continues Tuesday
-

 Local News4 days ago
Local News4 days agoSherral’s Diner to be featured on America’s Best Restaurants
-

 Mississippi Today7 days ago
Mississippi Today7 days agoOn this day in 1972
-

 News from the South - Georgia News Feed3 days ago
News from the South - Georgia News Feed3 days agoJose Ibarra found guilty in murder of Laken Riley | FOX 5 News
-
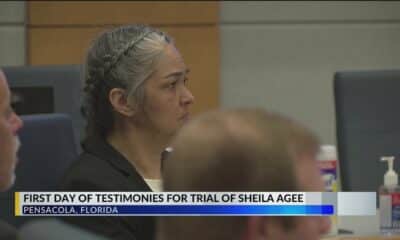
 News from the South - Alabama News Feed4 days ago
News from the South - Alabama News Feed4 days agoTrial underway for Sheila Agee, the mother accused in deadly Home Depot shooting
-

 News from the South - Kentucky News Feed3 days ago
News from the South - Kentucky News Feed3 days agoNicholasville organization activates weather plan in response to bitter cold temperatures
-

 News from the South - Alabama News Feed3 days ago
News from the South - Alabama News Feed3 days agoAlabama's weather forecast is getting colder, and a widespread frost and freeze is likely by the …
-

 News from the South - Alabama News Feed3 days ago
News from the South - Alabama News Feed3 days agoJudge grants mistrial in Sheila Agee trial due to ‘unhinged juror’