Kaiser Health News
The Drug Company That Prospered Without Creating Any Drugs
by Arthur Allen
Thu, 13 Apr 2023 09:00:00 +0000
The new drug looked so promising — except for that one warning sign.
At the American College of Rheumatology’s annual meeting in 2008, Duke University’s Dr. John Sundy proudly announced that pegloticase, a drug he’d helped develop, was astoundingly effective at treating severe gout, which affects perhaps 50,000 Americans. In about half of those who had taken it, the drug melted away the crystalline uric acid deposits that encrusted their joints to cause years of pain, immobility, or disfigurement.
But Sundy also disclosed an unsettling detail: In one clinical trial, patients who got the drug were more likely to develop heart problems than those who didn’t. The day after Sundy’s talk, the stock price of Savient Pharmaceuticals, which developed the drug with Duke scientists, plunged 75%.
That danger signal would disappear in later studies, and the FDA approved pegloticase, under the trade name Krystexxa, two years later. But the small biotech company never recovered. In 2013, Savient was sold at auction to Crealta, a private equity venture created for the purpose, for $120 million.
Two years later, a young company now called Horizon Therapeutics bought Crealta and its drug portfolio for $510 million.
Even at that price, it proved a good deal. Krystexxa brought in $716 million in 2022 and was expected to earn $1 billion annually in coming years.
Although Horizon says it now has 20 drugs under development, in its 15 years of existence it has yet to license a product it invented. Yet the company has managed to assemble a war chest of lucrative drugs, in the process writing a playbook for how to build a modern pharmaceutical colossus.
As the White House and both parties in Congress grapple with reining in prescription drug prices, Horizon’s approach reveals just how difficult this may be.
Horizon’s strategy has paid off handsomely. Krystexxa was just one of the many shiny objects that attracted Amgen, a pharmaceutical giant. Amgen announced in December that it intends to buy Horizon for $27.8 billion, in the biggest pharmaceutical industry deal announced in 2022.
Horizon’s CEO, Tim Walbert, who will reportedly get around $135 million when the deal closes, has mastered a particular kind of industry expertise: taking drugs invented and tested by other people, wrapping them expertly in hard-nosed marketing and warm-hued patient relations, raising their prices, and enjoying astounding revenues.
He’s done this with unusual finesse — courting patients with concierge-like attention and engaging specialist clinicians with lunches, conferences, and research projects, all while touting his own experience as a patient with a rare inflammatory disease. Walbert’s company has been particularly adept at ensuring that insurers, rather than patients, bear the costly burdens of his drugs.
A federal prosecutor in 2015 began examining allegations that Horizon’s patient assistance program had worked with specialty pharmacies to evade insurers’ efforts to shun Horizon’s expensive drugs. A separate probe opened in 2019 over alleged kickbacks to pharmacy benefit managers, companies that negotiate to get Horizon’s drugs covered by insurers. Those investigations appear to be no longer active, Horizon spokesperson Catherine Riedel said. The company this year disclosed a third probe, concerning methods the company allegedly used to get prior authorization of its drugs. Justice officials did not respond to requests for comment on the investigations.
An Injection of Marketing
To help sell its drugs, Horizon blankets specialist physicians with marketing and peer-to-peer appeals. Its payments to physicians for things like consulting, speeches, and meals totaled $8.7 million in 2021, compared with the $10 million it paid them for research, federal records show. By contrast, Seagen, a biotech company of roughly the same size, paid doctors a total of $116 million, with nearly $112 million of that pegged for research. Riedel said Horizon’s marketing and educational approaches were “necessarily unique” because of the challenges of treating rare and neglected diseases.
Walbert launched Horizon in 2008 in the Chicago area by combining and refashioning generic drugs into single pills. Duexis, Horizon’s first drug, is a mixture of generic Motrin and Pepcid. Its Vimovo combines generic Aleve and Nexium. In a 2017 article, a ProPublica reporter described being prescribed Vimovo for a shoulder injury. It cost him nothing, but his insurer was billed $3,252 for pills that together cost about $40 for a month’s supply in generic form. Horizon sold more than $57 million worth of Vimovo that year.
In 2014 and 2015, respectively, Horizon picked up two relatively new drugs that had no generic versions: the immunosuppressant Actimmune and Ravicti, which treats a rare genetic disorder. Soon Horizon was charging more than $50,000 a month for each, placing Actimmmune fourth and Ravicti second on GoodRx’s 2020 list of the most expensive U.S. drugs.
Horizon’s net sales soared from $20 million in 2012 to $981 million in 2016; Walbert’s pay package followed suit, topping an astronomical $93.4 million in 2015 in salary and stock. Stock analysts questioned the long-term soundness of a strategy of simply selling old drugs for mind-boggling prices, but Walbert was using the cash to refashion the company as a rare-diseases franchise.
His approach would make Walbert a darling of pharmaceutical investors and his board, which lavished him with over $20 million in compensation each of the past three years. While most biotechs and startups borrow heavily from venture capital to do science and have no idea how to develop and market a drug, Walbert got cash coming in quickly. “He did it backwards,” said Annabel Samimy, an analyst at Stifel Financial Corp. “Horizon built commercial platforms before they got into drug development.”
Generating “robust sales of what sounded like not very interesting drugs” allowed Walbert “to start a company on not very much,” said Oppenheimer analyst Leland Gershell. All the while, Horizon funded and cultivated the patient advocacy groups that can help lobby for a drug to be approved by the FDA and placed on insurers’ formularies, the lists of drugs health plans cover for patients.
Capitalizing on His Own Illness?
As Walbert and his spokespeople often point out, Walbert and his youngest son suffer from a rare disease, and Walbert also has an autoimmune disease. Walbert won’t name the diseases, but has said he’s taken the anti-inflammatory injectable Humira since 2003 — the year he led that drug’s commercial launch as a vice president at Abbott Laboratories. Humira has become the bestselling drug in history, with about $200 billion in all-time global sales.
In 2014, Walbert moved Horizon’s headquarters to Ireland, which nearly halved its tax rate. A year later it gained control of Krystexxa, and in 2017 it bought, for $145 million, a failing company that produced Tepezza, a drug for thyroid eye disease, which causes unsightly eye bulging and pain.
Tepezza quickly became a blockbuster, with $3.6 billion in total sales in 2021 and 2022. The company conducted additional clinical research on both Tepezza and Krystexxa, but it also spent heavily promoting these and other drugs to specialists who could prescribe them.
All the while it steadily raised prices. Savient put Krystexxa on the market in 2011 at $2,300 per injection. Horizon charges roughly 10 times as much. Six months of Tepezza treatment can run more than $400,000.
Horizon’s publicity emphasized the company’s sensitivity to patients, and its constant contact with disease advocates.
“Our scientists are attuned to the unmet needs of patients, their diagnostic and therapeutic journey,” Bill Rees, Horizon’s vice president for translational sciences, told KFF Health News. “It’s the marrying of the basic clinical science with a focus on the needs of the patient that differentiates us.”
To make sure patients keep using its drugs, clinicians say, Horizon staffers negotiate with insurance carriers, and the company offers drug discounts to lower-income patients while swaddling them with attention from its medical staff.
“Horizon has a nurse talk to each and every patient before every appointment,” said Dr. Brigid Freyne, who treats around half a dozen patients each year with Krystexxa at her Murrieta, California, rheumatology clinic. “The patients who come in here are highly motivated to get their IV. They get the message that it’s very important and they are fortunate to get the medicine.”
None of the manufacturers of her other infusion drugs shower patients with this kind of attention, she said.
While at Abbott, Walbert pioneered direct-to-consumer advertising for specialty drugs like Humira, a trend that aggravated insurers, who anticipated, correctly, that they would soon be shelling out billions for expensive drugs.
Horizon’s marketing plan for Krystexxa includes direct-to-consumer ads aimed at driving patients to specialists. The drug is designed for recalcitrant gout patients, who often have large lumps on their fingers, feet, and kidneys. Many, though not all, are heavy drinkers of beer or soda sweetened with high-fructose corn syrup, which can increase the buildup of uric acid, the cause of gout, said Dr. Robert McLean of Yale University.
While Krystexxa can help patients with advanced gout, the American College of Rheumatology views it as a drug of last resort, with plenty of cheaper, early intervention alternatives available.
“I prescribe it maybe once a year,” McLean said. “From a cost-effectiveness standpoint, it warrants questioning.”
Horizon recently started a publicity campaign addressed to all gout sufferers, urging them to see a rheumatologist or a nephrologist — the specialists it has targeted with Krystexxa educational materials — before the disease does too much harm.
“Horizon would like you to say, ‘Everyone with serious gout should be started on Krystexxa,’” said Dr. James O’Dell, a rheumatologist at the University of Nebraska Medical Center. The Horizon pitchmen he deals with are “nice guys, but we don’t believe that’s the best way.”
The company defends its marketing practices. “We learn what matters most to patient communities and act. This approach has been validated by independent third-party research,” said Riedel.
The Federal Trade Commission said in January it was seeking more information on the Amgen-Horizon merger. Sen. Elizabeth Warren (D-Mass.), citing high prices for Horizon and Amgen drugs, urged the agency to nix the deal.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
By: Arthur Allen
Title: The Drug Company That Prospered Without Creating Any Drugs
Sourced From: kffhealthnews.org/news/article/horizon-therapeutics-kyrstexxa-orphan-drug-profits/
Published Date: Thu, 13 Apr 2023 09:00:00 +0000
Did you miss our previous article…
https://www.biloxinewsevents.com/the-rate-of-older-californians-dying-of-malnutrition-has-accelerated/
Kaiser Health News
Anti-Fraud Efforts Meet Real-World Test During ACA Enrollment Period
SUMMARY: Unauthorized switching of Affordable Care Act (ACA) plans has decreased, with complaints dropping by a third, according to federal regulators. The Centers for Medicare & Medicaid Services (CMS) has implemented measures to combat fraud, such as requiring three-way calls for certain policy changes and suspending 850 agents involved in improper switching. Despite these efforts, concerns remain about increased complexity in the enrollment process, with potential delays for consumers. Some rogue agents are finding workarounds, and agent groups advocate for two-factor authentication to further prevent fraud. The CMS reports success in reducing unauthorized switches, but challenges remain.
The post Anti-Fraud Efforts Meet Real-World Test During ACA Enrollment Period appeared first on kffhealthnews.org
Kaiser Health News
Readers Embrace ‘Going It Alone’ Series on Aging and Chastise Makers of Pulse Oximeters
SUMMARY: Letters to the Editor discuss various healthcare concerns. Gail Daniels shares her struggles caring for a mother with dementia, while Shava Nerad reflects on the challenges faced by those without family support. Gloria Rankin suggests using pen pals to combat social isolation. Zoe Joyner Danielson recalls racial bias in pulse oximeter development, while Suzann Lebda questions fluoride’s impact on dental health. Readers also address issues like Medicare Advantage, high drug costs for seniors, and the financial burden of prepaying for baby deliveries. Liviu Steier advocates for fluorescence in dental care, emphasizing its diagnostic benefits.
The post Readers Embrace ‘Going It Alone’ Series on Aging and Chastise Makers of Pulse Oximeters appeared first on kffhealthnews.org
Kaiser Health News
Georgians With Disabilities Are Still Being Institutionalized, Despite Federal Oversight
SUMMARY: Lloyd Mills, a 32-year-old with autism, cerebral palsy, and kidney disease, has faced prolonged hospitalization due to inadequate community support in Georgia. After being admitted to Grady Memorial Hospital for mental health issues, Mills waited over eight months for appropriate housing, highlighting the systemic failures of a state still grappling with the consequences of a 2010 Department of Justice lawsuit regarding care for people with developmental disabilities. Despite significant investments and improvements in services, challenges like workforce shortages and inadequate funding persist, often leaving individuals like Mills in hospitals, impacting their mental and physical well-being.
The post Georgians With Disabilities Are Still Being Institutionalized, Despite Federal Oversight appeared first on kffhealthnews.org
-

 Local News6 days ago
Local News6 days agoSherral’s Diner to be featured on America’s Best Restaurants
-

 Local News3 days ago
Local News3 days agoIntroducing our Student Athlete of the Week: Ocean Springs’ very own Mackenzie Smith
-

 News from the South - Georgia News Feed6 days ago
News from the South - Georgia News Feed6 days agoJose Ibarra found guilty in murder of Laken Riley | FOX 5 News
-
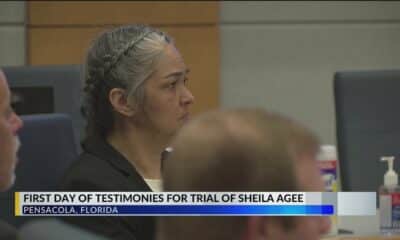
 News from the South - Alabama News Feed6 days ago
News from the South - Alabama News Feed6 days agoTrial underway for Sheila Agee, the mother accused in deadly Home Depot shooting
-

 News from the South - Kentucky News Feed5 days ago
News from the South - Kentucky News Feed5 days agoNicholasville organization activates weather plan in response to bitter cold temperatures
-

 News from the South - Alabama News Feed5 days ago
News from the South - Alabama News Feed5 days agoJudge grants mistrial in Sheila Agee trial due to ‘unhinged juror’
-

 News from the South - Alabama News Feed6 days ago
News from the South - Alabama News Feed6 days agoAlabama's weather forecast is getting colder, and a widespread frost and freeze is likely by the …
-

 News from the South - Louisiana News Feed7 days ago
News from the South - Louisiana News Feed7 days agoLawmakers discuss how music industry can improve Louisiana’s economy | Louisiana